About us
Our Objectives

We all need support through the most important period of our children: the early years
Aliquam rhoncus mauris, convallis volutpat velit bibendum dui duis ut vulputate amet, ac ipsum nisl convallis ut.
Pulvinar ultrices porta mattis quis lobortis est facilisis purus nunc, sed semper enim dictum sed donec condimentum sodales sed non vel malesuada morbi arcu justo, pretium sagittis hac nisi amet, fermentum nunc.
Blandit auctor felis habitasse aliquet est potenti ut urna eget orci pellentesque commodo vitae.
Founder & Head of School
Magna et nibh quam eu at viverra ut hac faucibus sed cras.
Felis mauris quisque scelerisque ac et, porta sit placerat pharetra, ac sodales vel vitae tincidunt mauris arcu placerat mi quis lorem orci, parturient rutrum.

Tortor platea nunc lorem morbi pellentesque sed enim viverra venenatis, sem pellentesque massa nunc quis lectus.
LUMINATE aims to:
Develop a first-of-its-kind arthroscopic 3D bioprinting tool (EndoFLight) for in situ tissue repair
Create bio-compatible photoresins tailored for cartilage and bone regeneration
Establish a one-stage regenerative approach using patient-derived and allogenic cells
Ensure clinical translation through regulatory compliance and market readiness
WP1: Clinical needs definition, GMP and regulatory conformity
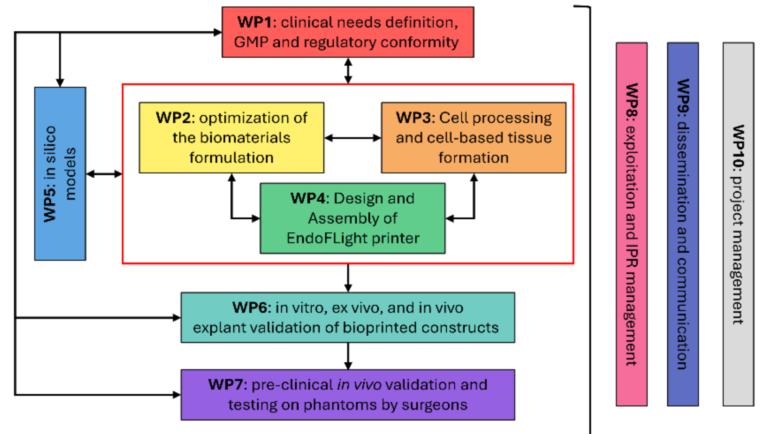
The LUMINATE project embarks on a journey to redefine osteochondral (OC) tissue regeneration, ensuring that patients suffering from knee cartilage and bone injuries receive groundbreaking, personalized treatment. Through its innovative in situ bioprinting technology, LUMINATE develops a minimally invasive, one-stage solution that could eliminate the need for traditional joint replacement surgeries. Each work package (WP) plays a crucial role in shaping this future, combining cutting-edge bioprinting, biomaterial engineering, artificial intelligence, and clinical validation to bring EndoFLight—the project’s revolutionary bioprinting suite—into reality.
WP2: Development of Novel Photoresins for Osteochondral Regeneration
At the heart of LUMINATE’s regenerative approach is the creation of bioengineered photoresins designed specifically for cartilage and bone repair. These biomaterials serve as the foundation for the bioprinted constructs that will integrate seamlessly into damaged joints. Researchers work tirelessly to develop resins that are biocompatible, mechanically strong, and capable of mimicking the native tissue environment.
For cartilage regeneration, the project develops a gelatin-hyaluronic acid-based bioresin that incorporates patient-derived chondrocytes and allogenic mesenchymal stromal cells (hMSCs) to promote rapid tissue healing. On the other hand, a bone-specific resin infused with nano-hydroxyapatite is designed to stimulate osseointegration, allowing bone tissue to regrow naturally. These materials must not only support cellular activity but also respond to precise light-based crosslinking processes that will occur within the body.
Through continuous formulation testing, rheological analysis, and in vitro validation, LUMINATE ensures that these bioresins are safe, effective, and scalable for clinical applications. By the project’s completion, these resins will be ready for regulatory approval and manufacturing under GMP guidelines, ensuring a seamless transition from research to real-world application.
WP3: Development
of EndoFLight Bioprinting Technology
A breakthrough in orthopedic surgery demands a radical shift in technology, and EndoFLight is at the core of this transformation. Designed to work within the body, this arthroscopic bioprinting unit enables surgeons to print living tissue directly into osteochondral defects in real time. The compact device is engineered with three innovative toolheads:
- Micro-extrusion, for precise deposition of bioresins into the defect.
- Filamented light projection, for instant crosslinking and structural reinforcement.
- Jetting technology, for delivering bioactive molecules that stimulate tissue growth and integration.
This state-of-the-art tool is more than a mechanical advancement; it is an intelligent, AI-enhanced surgical assistant. With an optical imaging system and real-time AI segmentation, EndoFLight analyzes the damaged tissue, calculates the precise amount of bioresin needed, and assists surgeons in delivering a patient-specific treatment. This innovation represents a seismic shift in how orthopedic conditions are treated, eliminating the need for multiple surgeries and lengthy rehabilitation periods.
WP4: In Silico Modeling & Optimization
Bringing bioprinting technology to life requires precise digital modeling and computational analysis. WP4 harnesses the power of artificial intelligence and advanced simulations to refine every aspect of LUMINATE’s bioprinting process.
Using AI-driven models, researchers simulate tissue behavior, optimize material deposition patterns, and predict the long-term biomechanical performance of printed constructs. This ensures that every bioprinted scaffold matches the complexity and durability of natural tissue. Furthermore, AI algorithms analyze patient-specific lesion data, automatically adjusting printing parameters to create truly personalized treatments.
By integrating machine learning and real-time imaging, LUMINATE ensures that each surgery is precise, predictable, and optimized for success. These in silico models drastically reduce development time and the need for costly experimental trials, accelerating the pathway toward clinical implementation.
WP5:
Preclinical Testing & Validation
As LUMINATE moves closer to human applications, rigorous preclinical testing becomes the final proving groundfor its bioprinting suite. WP5 is dedicated to evaluating the safety, efficacy, and biological integration of EndoFLight’s bioprinted constructs.
Initial tests focus on in vitro cell cultures and tissue explants, ensuring that the bioresins support cellular activity and matrix formation. Moving forward, the project scales up to animal models, where small-animal studies (mice) help determine early-stage biological responses, while large-animal models (sheep) allow full-scale evaluation of joint repair in a human-relevant setting.
Through a combination of histological analysis, biomechanical testing, and imaging techniques such as MRI and micro-CT, researchers assess:
- The structural fidelity of bioprinted cartilage and bone.
- The rate of cellular integration and tissue maturation.
- The mechanical resilience of the regenerated tissue under load-bearing conditions.
These findings will be essential in demonstrating that LUMINATE’s technology is safe, effective, and ready for the next step—clinical trials.
WP6: GMP Standardization & Regulatory Compliance
A revolutionary medical innovation is only valuable if it can reach patients and surgeons worldwide. WP6 is responsible for ensuring that EndoFLight and its bioresins meet the highest standards of medical safety, quality, and manufacturability.
Researchers work closely with regulatory bodies, including the European Medicines Agency (EMA) to establish a clear path to CE certification and clinical approval. Every component of the bioprinting suite—from the hardware to the bioresins and AI software—must comply with Medical Device Regulation (MDR 2017/745) and Good Manufacturing Practice (GMP) standards.
This WP also focuses on scaling up production, developing standardized protocols for large-scale bioresin manufacturing and ensuring that clinical-grade materials are ready for use in hospitals. By the end of the project, LUMINATE will have:
✔️ A fully compliant dossier for regulatory approval.
✔️ GMP-certified protocols for manufacturing bioresins and bioprinting hardware.
✔️ A roadmap for the first clinical trials to bring this technology to orthopedic patients.
WP7: Surgeon Training & Clinical Implementation
Even the most advanced medical technology requires skilled hands to bring it to life. WP7 ensures that surgeons across Europe are prepared to integrate LUMINATE’s bioprinting approach into their practice.
Through a combination of VR/AR training simulations, hands-on workshops, and real-world surgical demonstrations, orthopedic specialists learn:
✔️ How to use EndoFLight effectively in arthroscopic procedures.
✔️ How to optimize bioprinting parameters for different types of osteochondral injuries.
✔️ How to integrate AI-driven guidance into surgical workflows.
By establishing best practices and clinical guidelines, LUMINATE guarantees that hospitals can seamlessly adopt this technology, paving the way for widespread implementation.
WP9: Sustainability
& Ethical Considerations
Beyond clinical impact, LUMINATE is committed to ensuring that its innovations are sustainable, ethical, and environmentally responsible. WP9 develops eco-friendly bioresins, minimizes medical waste through on-demand in situ printing, and promotes ethical AI usage in healthcare.
Additionally, gender-specific differences in cartilage and bone regeneration are investigated, ensuring that treatments are optimized for both male and female patients. The project also actively engages with patient advocacy groups and regulatory policymakers, reinforcing the social responsibility of this groundbreaking work.
With each WP contributing critical advancements, LUMINATE is set to transform the way osteochondral injuries are treated. Through bioprinting innovation, AI-driven precision, and patient-centered solutions, the project paves the way for a future where damaged joints are no longer a lifelong burden—but a condition that can be healed from within.
What parent say

More than just a joyful place
Egestas pulvinar phasellus id odio viverra pharetra congue est eleifend aenean cras
