The LUMINATE project is dedicated to pioneering a transformational approach to osteochondral (OC) tissue regeneration, addressing a critical medical challenge in orthopedic healthcare. By leveraging state-of-the-art 3D bioprinting technology, LUMINATE aims to introduce a minimally invasive, patient-specific treatment that enhances healing, reduces surgical burden, and improves patient outcomes.
A core objective of LUMINATE is to develop EndoFLight, a multimaterial, multiscale 3D bioprinting device designed to work arthroscopically — directly within a patient’s joint during a single surgical procedure.
This innovative tool will:
By making in situ bioprinting a reality, LUMINATE eliminates the need for traditional tissue grafts, enabling a faster, more natural healing process.

The use of these bioengineered materials will provide a safer, more effective alternative to current cartilage replacement techniques, avoiding complications associated with synthetic implants.
Currently, most regenerative treatments for osteochondral injuries require multiple surgeries and long recovery periods. LUMINATE is pioneering a one-stage surgical approach, where:
This minimally invasive, personalized treatment significantly reduces recovery times, lowers healthcare costs, and improves long-term outcomes for patients.
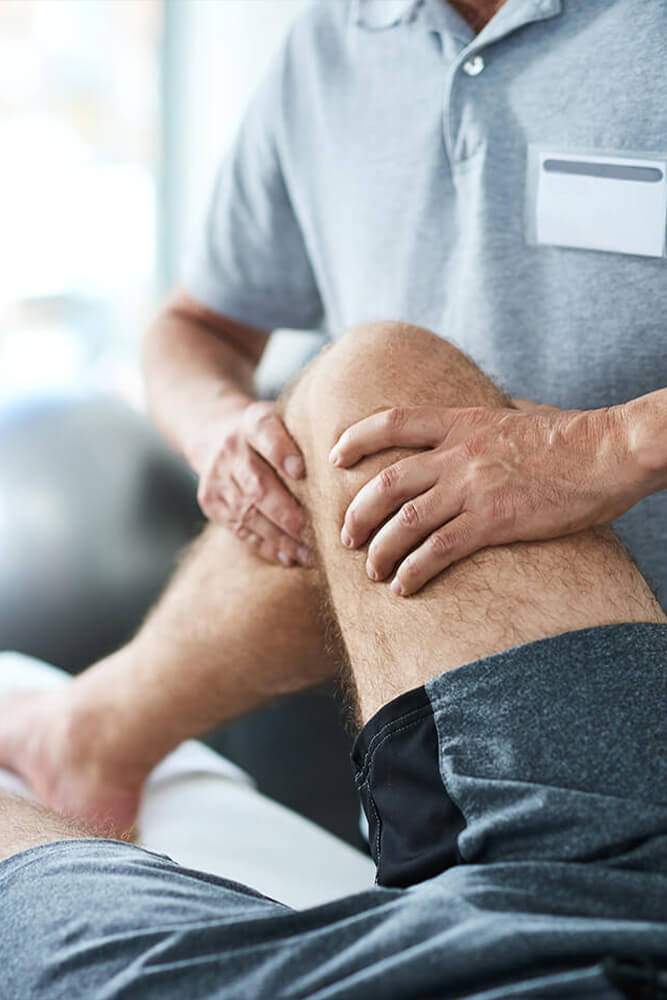
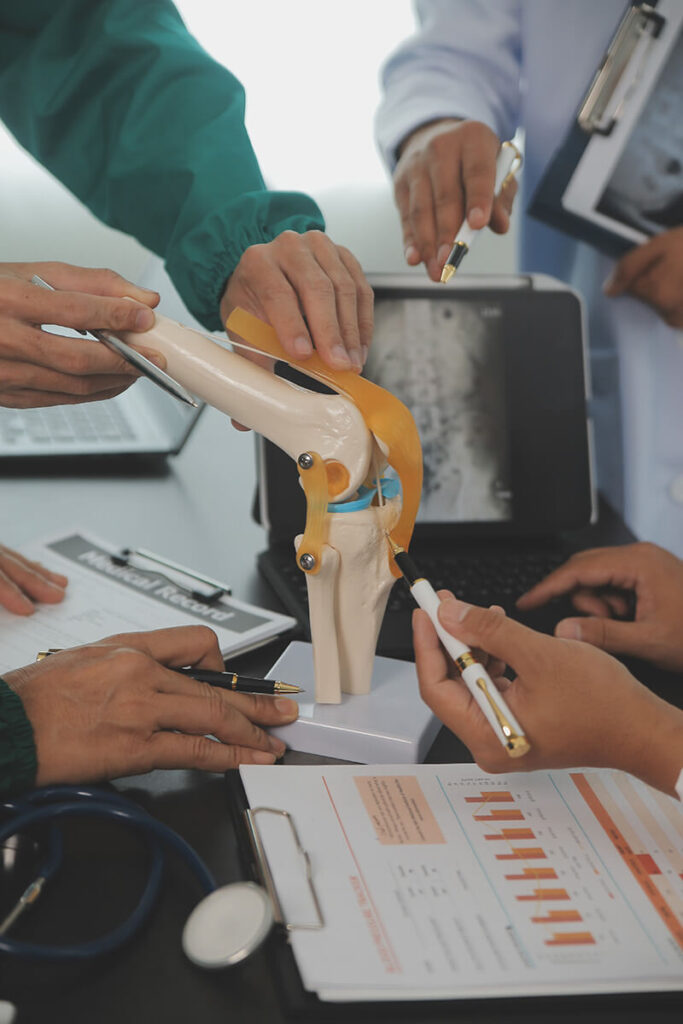
A key goal of LUMINATE is to ensure that its innovations reach real-world medical applications. The project is actively working on:
By focusing on clinical translation, LUMINATE ensures its breakthrough technology can be widely adopted by orthopedic surgeons and hospitals worldwide.
Beyond osteochondral repair, LUMINATE’s bioprinting technology holds far-reaching potential for other medical applications, including:
By pushing the boundaries of bioprinting, LUMINATE is laying the groundwork for a new era of regenerative medicine, where tissue restoration is faster, safer, and more effective than ever before.

LUMINATE promotes a sustainable, in situ bioprinting approach that significantly reduces the environmental footprint of orthopedic treatments.
Instead of relying on metallic implants, LUMINATE develops photo resins derived from natural biomaterials, reducing synthetic waste
Less hospitalization and post-surgical waste, compared to traditional joint replacements
Metal-based implants require energy-intensive extraction & processing. LUMINATE’s bioengineered tissues naturally integrate into the body, eliminating the need for hardware replacements
Unlike mass-produced implants, on-demand bioprinting minimizes the need for transportation, packaging, and storage, reducing carbon emissions.